
* The 18 groups in the periodic table are numbered from 1 to 18 according to IUPAC convention. * The Lanthanides and actinides are placed below the periodic table separately. Note: Actinium is also considered to be the part of f-block since its properties resemble more to actinoids. The names of some of its elements are not yet finalized (like Uut, Uup etc.) 7 Until recently this period is considered to be incomplete.

In this period, the 7s & 5f orbitals are filled up.
#Modern periodic table pdf series
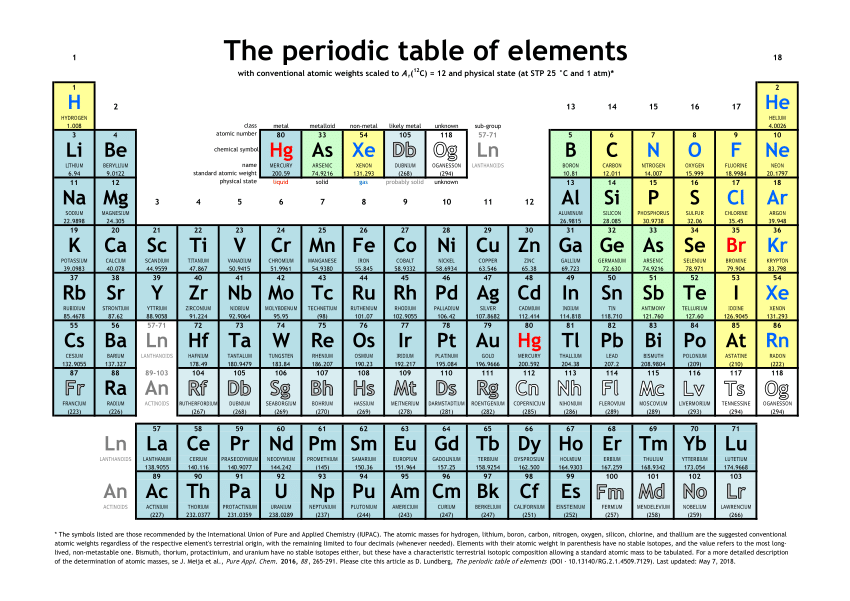
It also includes the 14 elements belonging to 5f series called actinides (Thorium (Th) to Lawrencium (Lr)). * The seventh period is an incomplete period. Note: Lanthanum is also considered to be the part of f-block since its properties resemble more to lanthanoids. In this period, the 6s & 6p along with 4f & 5d orbitals are being filled up. It not only includes 10 elements belonging to 5d series i.e., Lanthanum (La), Hafnium (Hf) to Mercury (Hg) but also contains 14 elements belonging the 4f series called lanthanides (Cerium (Ce) to Lutetium (Lu)). * The sixth period is the longest period with 32 elements. from Yttrium (Y) to Cadmium (Cd). The 5s & 5p along with 4d orbitals are filled up by electrons. It also includes 10 elements belonging to 4d series i.e. * The fifth period is the second long period with 18 elements, it starts with Rubidium (Rb) and ends with Xenon (Xe). It also includes 10 elements belonging to 3d series i.e., from Scandium (Sc) to Zinc (Zn). In this period, not only 4s & 4p and also the 3d orbitals are being filled up by electrons. * The fourth period is the first long period with 18 elements, it starts with Potassium (K) and ends with Krypton (Kr). It is called second short period. The 3s & 3p orbitals are being filled up in this period. * The third period also contain 8 elements i.e., from Sodium (Na) to Argon (Ar). It is called first short period. In this group, the 2s & 2p orbitals are being filled up. * The second period starts with Lithium (Li) and ends with Neon (Ne) and contains 8 elements. * The first period is a very short period with only two elements i.e., Hydrogen (H) & Helium (He). In this period, the 1s orbital is being filled up. * Each period starts with an alkali metal and ends with an inert gas element. The long form of modern periodic table consists of seven rows called periods and eighteen columns called groups. SALIENT FEATURES OF LONG FORM OF MODERN PERIODIC TABLE * The elements in a vertical column called as group should get similar outer electronic configuration since it is observed that the elements with similar outer electronic configuration show similar chemical properties. * Every row, also called as period, in the periodic table starts with the filling up of differentiating electron into a new quantum shell. * The elements in the periodic table are arranged in the increasing order of the atomic number. The following points are considered while constructing the periodic table. The modern long form of periodic table was constructed based on above law. "The chemical and physical properties of elements are the periodic functions of their atomic numbers and electronic configurations." Thus the modern periodic law can be stated as: The number of electrons in an atom and its electronic configuration are in turn are related to the atomic number. It was also found that there is a relation between electronic configuration and properties of elements. Later on it was clearly established that an element can be characterized by its atomic number, Z and not by the atomic weight. Where a & b are constants, characteristic of elements. The mathematical relation can be presented as: He found the relation between atomic numbers (Z) and the frequencies ( ν) of X-rays produced when the atoms of different elements are bombarded with cathode rays. The relation between the square root of frequency (√ ν) of highest energy emission line, called K α line, with the atomic number, Z was found to be linear. The modern periodic law was proposed by Moseley.


 0 kommentar(er)
0 kommentar(er)
